For Sales
From
Description
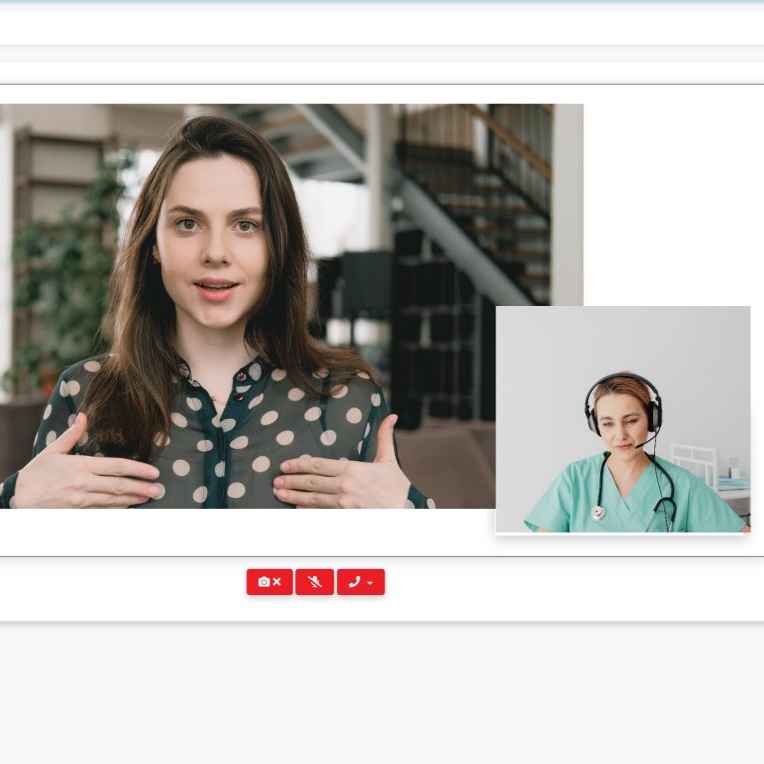
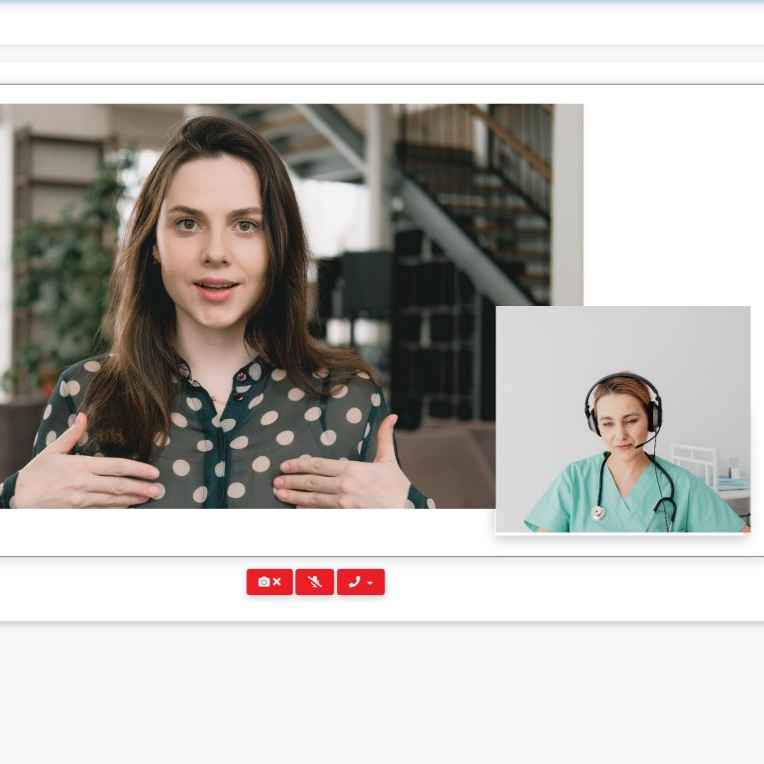
Clinical StudyPal’s eConsent feature automates the patient enrollment process and on-boards patients faster.
The importance and benefits of eConsent include, but are not limited to:
-Efficient use of time via automation, leading to a reduction of costs
-Automation also speeds up the study’s start-up process
-Reduces informed consent errors
-Unique ability to consider multiple government regulations
-Ability to configure for multiple language, increases the chances to reach a diverse population
-Remote consent monitoring/remote patient monitoring
-Creates a seamless and user-friendly experience, increasing patient compliance and patient retention
-Guaranteed signature compliance
-Allow patients to provide a hand signature or video consent
-eConsent can lead to a transformative experience for study participants by providing simple, easy-to-comprehend clinical trial information, which will boost patient engagement and therefore improve the patient experience overall.
